Mo Diagram Of Co Molecule Carbon Monoxide Molecular Orbital
Molecular orbital diagram of carbon monoxide co ( heteroatomic molecule C2 2 molecular orbital diagram Co lewis structure hybridization molecular geometry and mo diagram
Oxygen Orbital Diagram
Molecule explain sarthaks Oxygen orbital diagram 12+ molecular orbital diagram of co
Mo diagram bond carbon monoxide diatomic diatomics structure nitrogen molecular orbital theory molecules bonding synthesis meta webbook electron lewis so
How to draw molecular orbital diagram for co2Diagram mo energy level D6.5 mos for heteronuclear diatomic molecules – chemistry 109 fall 2021Molecular orbital diagram for co diagram media.
Molecular orbital diagram of co and noCarbon monoxide molecular orbital diagram Carbonyls compounds orbitals bond classnotesMo diagram and lewis structure of co.

Carbon dioxide molecular orbital diagram
Draw an mo energy diagram for coDraw an mo energy diagram for co What is the bond order of po?Metal carbonyls.
Draw mo diagram of co and calculate its bond order.Mo orbital diagram Orbital molecular monoxide bondsOrbital energy diagram for carbon.
Simplified occupancy orbital orbitals frontier bonding
7) the mo diagram for the co molecule is as follows:Mo diagram Mo diagram and characteristics of co moleculeExplain the mo diagram for no molecule..
(left) simplified mo diagram of co with electronic occupancy in theCarbonyls compounds orbitals bond classnotes Molecular orbital diagram of co moleculeDraw the molecular orbital energy diagram for co to predict the bond.

Molecular orbital theory energy level diagram for molecular orbitals
Mo diagram of coCarbon monoxide molecular orbital diagram Metal carbonylsDraw the molecular orbital diagram for co. based on your diagram, why.
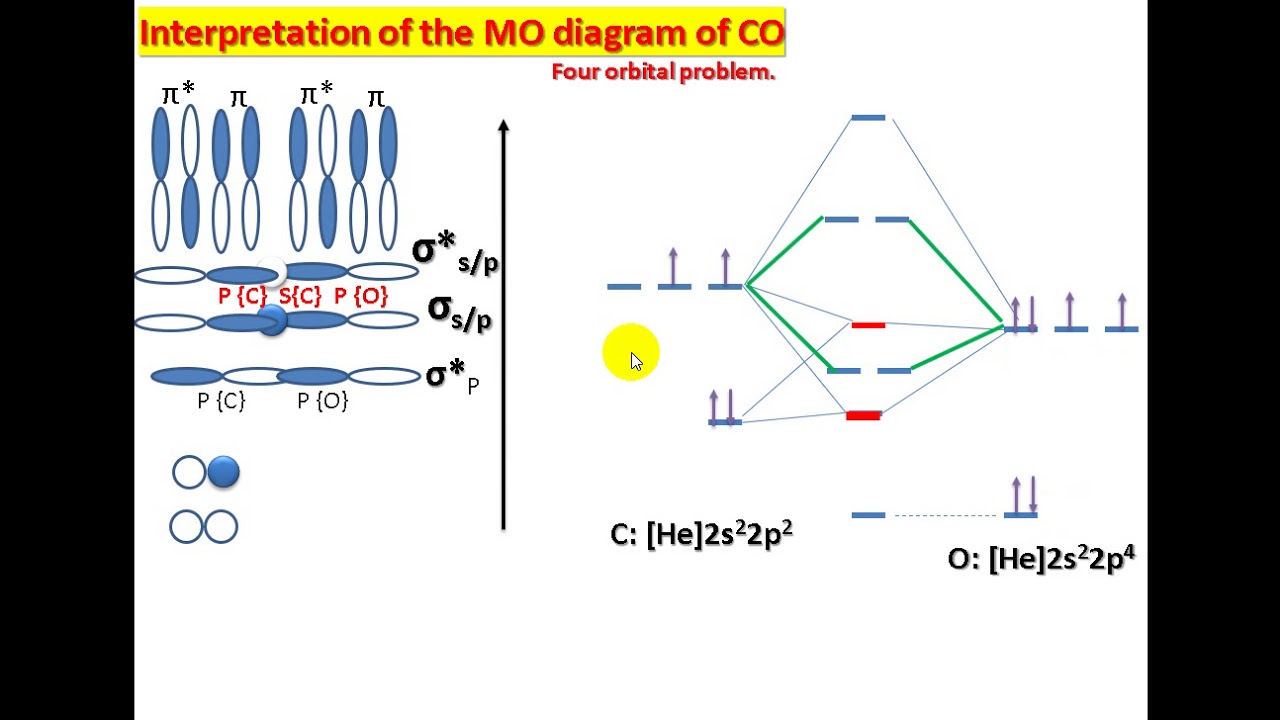
Interpretation of the mo diagram of coMo diatomic heteronuclear molecules orbital energy molecule chemistry Carbon monoxide molecular orbital diagramUse the drawing of mo energy diagram of co to predict the bond order.

Solved 10. (10 pooints) the molecular orbital energy level
.
.